Evaluation on the effect of local and imported yeast as supplementary feed on African catfish (Clarias gariepinus Burchell, 1822) in Egypt 
2. National Institute of Oceanography and Fisheries; Alexandria- Egypt
 Author
Author  Correspondence author
Correspondence author
International Journal of Aquaculture, 2015, Vol. 5, No. 25 doi: 10.5376/ija.2015.05.0025
Received: 04 May, 2015 Accepted: 05 Jun., 2015 Published: 18 Aug., 2015
Mona M. H., Alamdeen A. A., Elgayar E. E., Heneish A. M and El-feky Mohamed M. M., 2015, Evaluation on the effect of local and imported yeast as supplementary feed on African catfish (Clarias gariepinus Burchell, 1822) in Egypt, International Journal of Aquaculture, 5(25): 1-8
This study aims to evaluate the effect of different graded levels of local and imported yeast (Saccharomyces cerevisiae) on growth performance of African catfish, Clarias gariepinus. A total of 250 fingerlings of the African catfish Clarias gariepnus were collected from Edku Lake for the experiment. The fish were divided into 5 groups, containing of 50 fish in each group. Describe the group and treatment given to each group of the fish. The results showed that the supplementation of local yeast, improved the growth and feed utilization. Significant results were recorded for treatment of G2 compared to the G1. It was shown that the yeast supplementation significantly affected the whole-fish body composition. All treatments exhibited higher values compared to the G1. Treatment of G2 also showed the lowest values for dray matter, ether extract and ash content, while it showed highest value for crude protein compared to the G1. Hematological analysis of all the treatments showed satisfactory values compared to the G1. From the economic point of view, the utilization of local baker's yeast for African catfish could reduce the cost, while increases the growth and production performance under farming conditions.
Introduction
The African catfish, (Clarias gariepinus Burchell, 1822), is a freshwater eurytopic fish species. The superior performance of this species compared to other Clarias species in terms of growth rate has probably contributed to fact that C. gariepinus has been widely introduced to areas outside its natural range (Verreth et al., 1993). Please add more information about the importance of this fish species.
Bakers’ yeast, Saccharomyces cerevisiae, is used in the bakers industry. This yeast contains various immunostimulating compounds such as β- glucans, nucleic acids as well as mannan oligosaccharides, and it has the capability to enhance immune responses (Ortuño et al., 2002) as well as growth of various fish species (Li and Gatlin, 2005).
The main objective of the present study was to evaluate the effects of different grad level of local and import yeast (Saccharomyces cerevisiae) as feed supplement on African catfish, in terms of their growth and production performance, feed utilization as well as hematological parameters.
Material & Methods
Fish culture and feeding regime
A total of 250 fingerlings of the African catfish (mean body weight 100 ± 0.67g) were collected from Edku Lake. The fish were then transported to the fish hatchery in El-Max Fish farm, National Institute of Oceanography and Fishers (NIOF), Alexandria, Egypt. After two weeks for acclimation, the fish were divided into 5 groups of 50 fish in each group. The fish then were kept in aerated rectangle fiber glass tanks (0.90 m x 3.70 m x 1.90 m) for acclimatization and during experimental trials (Figure 1). The tanks were filled with tap water where the dissolved oxygen were maintained at 5.6 g/l±SD?, pH at 7.9±SD?, water temperature at 26-27°C, and photoperiod was set up at 12:12 (light: dark).
The five fish groups were exposed to different five diets. Group 1 (G1) was fed on commercial pellets diet without yeast and acts as a control, group 2 (G2) was fed on commercial pellets diet with 2% local Baker Yeast (Saccharomyces cerevisiae), group 3 (G3) was fed on commercial pellets diet with 4% local Baker Yeast (Saccharomyces cerevisiae), group 4 (G4) was fed on commercial pellets diet with 2% imported Tonilisite (Saccharomyces cerevisiae) and group 5 (G5) was fed on commercial pellets diet with 4% imported Tonilisite (Saccharomyces cerevisiae).
The feeding rate was 3% of the biomass and given twice a day, at 10.00 AM and 2.00 PM, for six days a week for a period of eight weeks. The composition and chemical analysis of the experimental pellets were measured and are presented in Tables 1 and 2. The diets were analyzed according to the standard methods of AOAC (1990). The experimental tanks were inspected daily to remove dead fish, if present.
Remove this figure
Chemical analysis of fish and diets
The whole-fish body and the tested diets were analyzed according to the standard methods of AOAC (1990) for moisture, crude protein, crude fat and ash. Moisture content was calculated by determining the differences before and after drying oven at 105ºC for 16 h. Nitrogen content was determined by the micro-Kjeldahl method. A factor of 6.25 was used to convert the nitrogen content to the crude protein. Crude fat was determined by drying the samples at 100ºC for 12 h and then extracting the crude fat with petroleum ether in soxhlet extractor for 4 h. Total ash was determined using muffle furnace at 550°C for 6 h.
Water quality analysis
Water samples were collected twice per week from each aquarium. Temperatures were measured on site with YSI model. The salinity was measured by using Salino-meter. Unionized ammonia was measured using Drel/2 Hach kits. The pH was measured using a pH-meter. All the water quality parameters were within
the acceptable ranges for the fish growth (Boyd, 1984).
Growth performance and survival rate
Weight gain (WG), average daily gain (ADG), percentage average daily gain (ADG %), specific growth rate (SGR %) and survival rate (S %) were calculated according to the following equation:
Gain (Gg) =final fish weight (g) – initial fish weight (g).
Gain % (G %) = Gain of fish (g) / initial weight of fish (g) X 100.
ADG = Gain (g) / time (DAY).
ADG % = {ADG / Initial weight of fish (g)} X 100.
SGR% = 100 X {(In W2 – In W1) / T}
Where. W2 is the final weight of fish (g).
Where. W1is the initial weight of fish (g).
In is natural log.
T is the time (day).
Survival rate (S %) was determinate as follows:
S % = Number of fish at the end / Total initial number of fish x100.
Feed utilization
Feed conversion ration (FCR) was calculated according to the following equation:
FCR =Feed intake (g) / Weight gain (g).
Hepatosomatic and gonadosomatic index determination
At the end of the experiment, all fish were sacrified. The liver and gonads were removed and weight to determine the hepatosomatic index (HSI) (Jangaard et al., 1967) and gonadosomatic index (GSI) (Tseng and Chan, 1982) as follow:
HSI = liver weight x 100/ fish weight
GSI = gonads weight x 100/ fish weight
Hematological and biochemical analyses
At the end of the experiment, blood samples were collected from the fish caudal peduncle of the different groups. Erythrocytes count (RBC) and total leukocytes count (WBC) were measured on an Ao Bright-Line Haemocytometer model (Neubauer improved, Precicolor HBG, Germany). Hemoglobin concentration (Hb gm/dl) was estimated according to the method of Zinkl (1986). Differential leukocyte count was estimated according to Vankamlen (1961).
The total protein (TP) concentration was measured according to the method of Henry, (1964). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were assayed according to the method of Reitman and Frankel (1957). Serum cholesterol (mg/dl) was estimated by enzymatic colorimetric methods. Triglyceride (mg/dl) was estimated according to the method of (Fridewald et al., 1972).
Statistical analysis
Data was subjected to one-way analysis of variance.
(ANOVA) followed by the Duncan's multiple comparison test for the means. The results are presented as mean ± SE . The results are significant at P≤0.05.
Results
Chemical analysis of fish
Yeast supplementation significantly affected whole-fish body composition especially moisture with the yeast treatments at different doses. Fish fed on the control diet (G1) had the lowest protein content (15.51±0.59). Yeast supplementation significantly improved the protein content and reach to 16.06±0.34 in the catfish treated with 2% local yeast (G2). However, treatment with 4% local yeast and 2% or 4% imported yeast had a non significant change in the protein content.
No significant difference in lipid content (Ether Extract) in fish body was observed between the control and treated group. Ash content had non significant increase in the fish in group G2, G3, G4 and G5 respectively. However, there is a significant increase in ash content in the fish of G5 as shown Table 3.
|
Table 3 Proximate chemical analysis of African catfish, moisture (M), crude protein (CP), ether extract (EE), and ash throughout the investigation period *- Significant difference (P≤0.05) between fish group or between proximate chemical analysis group? |
Quality parameters of rearing water
The present data showed that, during the experiment, there is a non significant change in the ammonia, salinity, pH and temperature values in the catfish of groups G2, G3, G and G5, respectively, compared with the G1 (Table 4).
|
Table 4 Means of water quality parameters, ammonia, salinity, hydrogen ion concentration (pH) and temperature during the experimental period |
Growth performance and survival rate
The results showed that there is a significant increase in the gain weight of the catfish of groups G2, G3, G4 and G5, respectively compared with the G1. In addition, there is a significant increase in the specific growth rate of the catfish treated with 2% local yeast (G2) compared with the G1. However, there is a non significant increase in the specific growth rate of the catfish treated with 4% local yeast (G3) or 2% and 4% imported yeast (G4 and G5) compared with the G1.
The present data showed that there is a significant decrease in the feed conversion ratio of the catfish treated with 2% local yeast (G2) compared with the G1. However, there is a non significant decrease in the feed conversion ratio of the catfish treated with 4% local yeast (G3) or 2% and 4% imported yeast (G4 and G5) compared with the G1The survival rate for the control and the treated groups was 100% (Table 5).
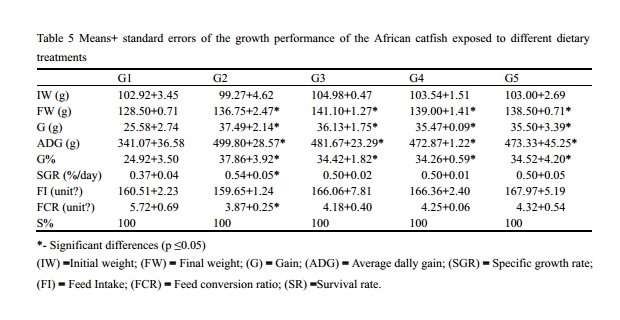 Table 5 Means+ standard errors of the growth performance of the African catfish exposed to different dietary treatments *- Significant differences (p ≤0.05) (IW) =Initial weight; (FW) = Final weight; (G) = Gain; (ADG) = Average dally gain; (SGR) = Specific growth rate; (FI) = Feed Intake; (FCR) = Feed conversion ratio; (SR) =Survival rate. |
Hepatosomatic and gonadosomatic indexThe data showed that there is a significant increase in the GSI of the male catfish treated with 2% local yeast (G2) and 4% local yeast (G3) compared with the G1. However, there is a non significant decrease in the GSIof the male catfish treated with or 2% imported yeast (G4) and a non significant increase in the GSI of the male catfish treated with 4% imported yeast (G5), respectively, compared with the G1. Furthermore, there is a significant increase in the GSI of the female catfish treated with 2% local yeast (G2), 4% local yeast (G3) and 2% imported yeast (G4), respectively, compared with the G1. However, there is a non significant increase = in the GSI of the female catfish treated with 4% import yeast (G5) compared with the G1.
On the other hand, there is a non significant change in the HSI of all treated groups (G2, G3, G4 and G5), respectively, compared with the G1 (Table 6).
|
Table 6 Means + standard errors of the internal organs induce of the catfish at the end of the investigation period as affected by the different yeast levels *- Significant differences (p ≤0.05) |
Hematological and Biochemical Analyses
Hematological parameters
The present data showed that there is a significant increase in the RBC of the catfish treated with 2% import yeast (G4) compared with the G1. However, there is a non significant change in the RBC of the catfish treated with 2% (G2), 4% local yeast (G3) and 4% imported yeast (G5) with the G1. However, there is a non significant change in the WBC, hemoglobin levels, deferential leucocytes count in all treated groups (G2, G3, G4 and G5), respectively, compared with the G1 (Table 7).
|
Table 7 Haematological parameters of catfish at different of yeast levels *- Significant differences (p ≤0.05) |
Biochemical parameters
The present data showed that there is a significant increase in the total protein of the catfish treated with 4% local yeast (G3) and 2% import yeast (G4) compared with the G1. However, there is a non significant increase in the total protein of the catfish treated with 2% local yeast (G3) or 4% imported yeast (G5) compared with the G1.
The data showed that there is a significant increase in the ASTof the catfish treated with 2% local yeast and 2% and 4% imported yeast (G2), (G4) and (G5), respectively, comparing with the G1. However, there is a non significant increase in the AST of the catfish treated with 4% local yeast (G3) compared with the G1. In addition, there is a significant decrease in the ALT of all treated groups (G2, G3, G4 and G5) receptively, compared with the G1.
The data showed that there is a significant decrease in the cholesterol level of the catfish treated with 2% local yeast (G2), 2% (G4) and 4% import yeast (G5) , and comparing with the G1. However, there is a non significant decrease in the cholesterol level of the catfish treated with 4% local yeast (G3) compared with the G1. In addition, there is a significant increase in the triglyceride level of the catfish treated with 2% local yeast (G2) compared with the G1. However, there is a non significant change in the triglyceride levels of the catfish treated with 4% local yeast and 2% and 4% import yeast (G3), (G4) and (G5), respectively, compared with the G1 (Table 8).
|
Table 8 Physiological parameters of catfish at different of yeast levels Stare: The significant difference at (P<0.05). No stare: no significant (P≥0.05) |
Discussion
The present study showed that yeast supplementation improved the protein content in the catfish that treated with 2% local yeast. However no significant difference in lipid or ash content in both controlled fish body either the fish that treat with either local or import yeast. Abdel (Tawwab et al., 2006) reported that changes in protein deposition and ash content in catfish body could be linked with changes in their synthesis, deposition rate in muscle and or different growth rate.
The present data showed that the physico-chemical parameters of water throughout the experiment were within the acceptable ranges recommended for the culture of African catfish, C. gariepinus with regard to the ammonia, salinity, pH and temperature values. This is in consistent with Abdelhamid (2009) who recorded that the physico-chemical parameters of water were within the acceptable ranges recommended for pisciculture especially the culture of African catfish, C. gariepinus
The present study showed that there is a significant increase in the body weight and the specific growth rate the catfish that treated with 2% local yeast. However there is a significant decrease in the feed conversion ratio of the catfish that treated with 2% local yeast. The present study confirmed the previous findings showing the positive effect of yeast on growth rate, feed conversion ratio and nutrient efficiency utilization of catfish (Kobeiusy and Hussein, 1995). (Rumsey et al., 1992) explained that the enhanced growth performance and feed utilization may be due to the live yeast act as a source of some enzymes, amylase, protease and lipase which may improve food digestion and consequently food utilization. (Lara-Flores et al., 2003) stated that the improvement of nutrient utilization and feed conversion ratio by using probiotic baker's yeast in African catfish diets may attributed to the act of the cell walls of yeast which provide very important non-nutritive compounds that may benefit fish health, including mannose. They added that the addition of live yeast improved diet and protein digestibility, which may explain the better growth and feed efficiency with yeast supplements. Therefore, from the previous results, it can be concluded that supplementation of a diet with a supplementation of a percentage of 2% of commercial local yeast probiotic could be beneficial for growth and survival of African catfish, especially in fast growing conditions, where it may be essential to stimulate the precocious of digestive system.
A significant increase in the GSI the stages of gonadal development observed in both male and female Pomadasys stridens in this study are according to Nikolsky (1963). However, Abd El-Hakim and El-Gamal, (2000) found that the lead acetate is associated with the decrease in both of GSI and health state of Oreochromis niloticus. Moreover, Hussein and Kobeisy (1999) reported that HSI of the fish, Oreochromis niloticus was not affected by oxygen deficiency.
A significant increase in the RBC of the catfish treated with 2% import yeast was observed. This result agrees with (Taoka et al., 2006b) who investigated the effect of live and dead probiotic cells on the non-specific immune system of Nile tilapia. Fish hematology is gaining great attention in fish culture because of its importance in monitoring the health status of fish (Hrubec et al., 2000). Bakers’ yeast is a source of nucleic acids and β-1, 3-glucans which have been recognized to effectively enhance immune functions of African catfish (Yoshida et al., 1995).
Moreover, a significant increase in the total protein of the catfish that treated with 4% local yeast or 2% imported yeast is observed. Plasma proteins were quantified according to Bradford (1976) with Comassie Brilliant Blue G-250 (Sigma) and using Bovine Serum Albumin (BSA) standards. In case of cultured species, occasionally health issues arise that necessitate clinical evaluation of the fish under such captive conditions. Lack of published species-specific normal reference ranges remains the primary reason that blood testing is not routinely performed in fish health evaluations (Mauel et al., 2007). The hematological characteristic is an important tool that can be used as an effective and sensitive index to monitor physiological and pathological changes in fish (Kori-Siakpere et al., 2005).
The data showed that there is a significant increase in the AST of the catfish treated with 2% local yeast and 2% and 4% imported yeast. In addition, there was a significant decrease in the ALT of all treated yeast. AST and ALT belong to the plasma non-functional enzymes which are normally localized within the cells of liver, heart, gills, kidneys, muscle and other organs (Carrillo et al., 1991). A significant in the cholesterol level of the catfish treated with 2% local yeast, 2% and 4% import yeast compared to what?. In addition, there was a significant in the triglyceride level of the catfish treated with 2% local yeast comparing with the control group. Cholesterol is the most important sterol occurring in plasma, but in adrenal cortex, it occurs in the esterifies form (Wendelaar Bonga, 1997).
Recommendation
The present study indicates that live yeast (Saccharomyces cerevisiae) positively enhance on feed utilization of catfish. However, no significant different? was found among local and imported live yeast, or between 2% and 4% of supplementation levels. Thus, the utilization of local baker's yeast at 2%was recommended in catfish feeds from economic point of view.
References
Abdel- Hakim, N. F. AND El Gamal, A.A. (2000): Culture of Nile tilapia (Oreochromis niloticus) in rice fish culture system. Conference of Social and Agriculture Development of Sinai ,D70-81
Abdelhamid, A. M. (2009): Fungi and Mycotoxin, 1st Ed. Dar Anashr for Universities, Cairo, Deposition No. 13738/97, ISBN: 977/5526180/9,539p.
Abdelhamid, A. M., Ahmed, A. M. and El-Meleigy, Kh. M. (2004): An attempt to alleviate the histological alterations of some internal organs of rats fed on aflatoxin contaminated diets. J. Agric. Sci. Mansoura Univ, 29: 2355-2370
Abdel-Tawwab, M., Khattab, Y. A. E., Ahmad, M. H. and Shalaby, A. M. E. (2006): Compensatory growth, feed utilization, whole-body composition and hematological changes in starved juvenile Nile tilapia, Oreochromis niloticus (L.). J. Appl. Aquac, 18, 17-36
http://dx.doi.org/10.1300/J028v18n03_02
Association of Analytical Chemists (A.O.A.C) (1990): Official methods of analysis, 16th edition. AOAC, Arlington, Virginia.
Boyd, C.E., (1984): Water Quality in Warm water Fishponds. Auburn University
Bradford, M.M., (1976): A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem, 72, 248-254
http://dx.doi.org/10.1016/0003-2697(76)90527-3
Carrillo, M., Zanuy, S., Kqhn, E.R., (1991): Seasonal changes in thyroid activity of male sea bass (Dicentrarchus labrax Linnaeus 1758) (Perciformes: Serranidae) adapted to different
Fagbenro, O. A., Balogun, B., Ibironke, N. and Fasina, F. (1993): Nutritional values of some amphibian in diets for Clarias gariepinus (Burchell, 1822) (Siluroformes: Clariidae). Journal of Aquaculture in the Tropics, 8: 95-101
Friedewald, W. T., Levy, R. A. and Fredrickson, D. S. (1972): Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem; 18:499-502
Fuller, R. (1989): Probiotics in man and animal. J. Appl. Bacteriol, 66, 365-378
http://dx.doi.org/10.1111/j.1365-2672.1989.tb05105.x
Henry, R. J. (1964): Colorimetric determination of total protein. In: Clinical Chemistry. Harper and Row Publ., New York, USA.
Hrubec, T. C., Cardinale, J. L. and Smith, S. A. (2000): Hematology and plasma chemistry reference Intervals for cultured tilapia (Oreochomis hybrid). Vet. Clin. Pathol, 29: 7-12
http://dx.doi.org/10.1111/j.1939-165X.2000.tb00389.x
Huisman, E. A. (1986): Current Status and Role of Aquaculture with Species Reference to the African region. Ln. Aquaculture Resaerch in the African Region. (E. A. Huisman Ed.) PUDOC. Wagenigen, the Netherlands, pp 11-12
Hussein, S. Y. and Kobeisy, M. A. (1999): Enfelunce of heat strees on growth performance and some blood constituents of Oreochromis niloticus fed ascorbic acid .Assuit Veterinary Medical J.,41 (8): 17-33
Jangaard, P.M. Ackman, R.G. and Spios, J. C., (1967): Seasonal studies of the fatty acids composition of cod liver flesh, roe and milt lipids.J. Fish Res. Bd. Of Canada, 24:613-627
http://dx.doi.org/10.1139/f67-053
Kesarcodi-Watson, A., Kaspar, H., Lategan, M. J. and Gibson, L. (2008): Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquac, 274, 1-14
http://dx.doi.org/10.1016/j.aquaculture.2007.11.019
Kobeiusy, M. A. and Hussein, S. Y. (1995): Influence of Dietary Live Yeast on Growth Performance and Some Blood Constituents in Oreochromis Niloticus. Proceedings of 5th science confertence Animal Nutrition, Dec. 12-13, Ismailia, Egypt, Pp: 417-425
Kori-Siakpere, O.; Ake, J.E.G. and Idoge, E. (2005): Haematological characteristics of the African snakehead, Parachanna obscura. Afr. J. Biotechnol., 4(6): 527-530.
Lara-Flores, M., Olvera-Novoa, M. A., Guzman-Méndez, B. E. and López- Madrid, W. (2003): Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and theyeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture, 216, 193-201
http://dx.doi.org/10.1016/S0044-8486(02)00277-6
Li, P. and Gatlin, D. M. (2005): Evaluation of brewers yeast (Saccharomyces Cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops_M. saxatilis) Aquac, 219; 681-692
http://dx.doi.org/10.1016/S0044-8486(02)00653-1
Mauel, M., Bénédicte, B., Rafael, L. and Christian, L. (2007): Rapid Synthesis and Synaptic Insertion of GluR2 for mGluR-LTD in the Ventral Tegmental Area American Association for the Advancement of Science, 1200 New York.
Nikolsky, G. V. (1963): The ecology of fishes. Academy Press, London
Ortuno, J., Cuesta, A., Rodríguez, A., Esteban, M. A. and Meseguer, J., (2002): Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet. Immunol. Immunopathol, 85, 41-50
http://dx.doi.org/10.1016/S0165-2427(01)00406-8
Reitman, S. and Frankel, S. (1957): A colourmetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol, 28, 56
Ringo, E., olsen, R. E., Gifstad, T. O., Damo, R. A., Amlund, H., Hemre G. I. and Bakke, A. M. (2010): prebiotics in aquaculture: Areview. Aquacult. Nutr., 16: 117-136
Rumsey, G. L., Winfree, R. A. and Hughes, S. G. (1992): Nutritional Value of Dietary Nucleic Acids and Purine Bases to Rainbow Trout (Oncorhynchus Mykiss). Aquac, 108: 97-110
http://dx.doi.org/10.1016/0044-8486(92)90321-B
Salem, M. E. M., (2008): Studies on some medicinal plants as anti mycotoxins in fish diets. M. Sc. Thesis, fac. Agri., Kaf. El-Sh. Univ. Egypt. 116pp.
Solomo, E. S. and Ezegbo, B. (2010): Growing and cultivation of different species of fish, 50: 5978-5986
Taoka, Y., Maeda, H., Jo, J.-Y., Kim, S.-M., Park, S., Yoshikawa, T., Sakata, T., (2006b): Use of live and dead probiotic cells in tilapia Oreochromis niloticus. Fish. Sci, 72, 755-766
http://dx.doi.org/10.1111/j.1444-2906.2006.01215.x
Teuber, M. (2001): Veterinary use and antibiotic resistance. Curr. Opin. Microbiol. 4, 493-499
http://dx.doi.org/10.1016/S1369-5274(00)00241-1
Thomas, J. Jerobin, J. Seelan, T. S. J. Thanigaivel, S. Vijayakumar, S. Mukherjee, A. and Chandrasekaran, N. (2013): Studies on pathogenecity of Aeromonas salmonicida in catfish Clarias batrachus and control measures by neem nanoemulsion Aquaculture, 396-399, 71-75
http://dx.doi.org/10.1016/j.aquaculture.2013.02.024
Tseng, W.Y. and Chan, K.L., (1982): The reproductive biology of the rabbit fish in hong kong .J. word Maricul .Soc. 13:31-321
Vankamlen, E. J. (1961): Clinical Chem. Acta, 6: 538-544
http://dx.doi.org/10.1016/0009-8981(61)90145-0
Verreth, J. A. J., Torreele, E., Spazier, E., Van Der, A., Rombout, J. H. W. M., Booms, R. and Segner, H. (1992): The development of a functional digestive system in the African catfish, Clarias gariepinus (Burchell). Jounal of the World Aquaculture Society, 23 (199): 286-298
http://dx.doi.org/10.1111/j.1749-7345.1992.tb00792.x
Verreth, J., Eding, E. H., Rao, G. R. M., Huskens, F. and Segner, H. (1993): A review of feeding practices, growth and nutritional physiology in larvae of the catfishes Clarias gariepinus and Clarias batrachus. Journal of the World Aquaculture Society, 24 (2): 135-144
http://dx.doi.org/10.1111/j.1749-7345.1993.tb00002.x
Verschuere, L., Rombaut G., Sorgeloos P. and Verstraete, W. (2000): Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev.; 64, 655-671
http://dx.doi.org/10.1128/MMBR.64.4.655-671.2000
Wendelaar Bonga, S.E., (1997): The stress response in fish. Physiol. Rev, 77 (3), 591-625
Yoshida, T., Kruger, R., Inglis, V., (1995): Augmentation of non-specific protection in African catfish, Clarias gariepinus (Burchell), by the long-term oral administration of immunostimulants. J. Fish Dis, 18, 195-198
http://dx.doi.org/10.1111/j.1365-2761.1995.tb00278.x
Zinkl, J. G. (1986): Avian hematology. In: Jain NC (Edd. Schalm’s Veterinary Hematology, Philadelphia, Pai hea and Febiger, pp. 256-260
. PDF(398KB)
. HTML
Associated material
. Readers' comments
Other articles by authors
. Mona M. H.
. Alamdeen A. A.
. Elgayar E. E.
. Heneish A. M.
. El-feky Mohamed M. M.
Related articles
. African catfish
. Supplementary food
. Growth and production
. Yeast
Tools
. Email to a friend
. Post a comment
.jpg)
.jpg)


